Healthcare solution
Monitor patients and collect 24/7 health data with access to high resolution physiological raw data, independent and secure hosting, survey and questionnaire responses, real-time data streams, health data reports and exports.
Secure Platform
GDPR Compliant
ISO Certified Cloud
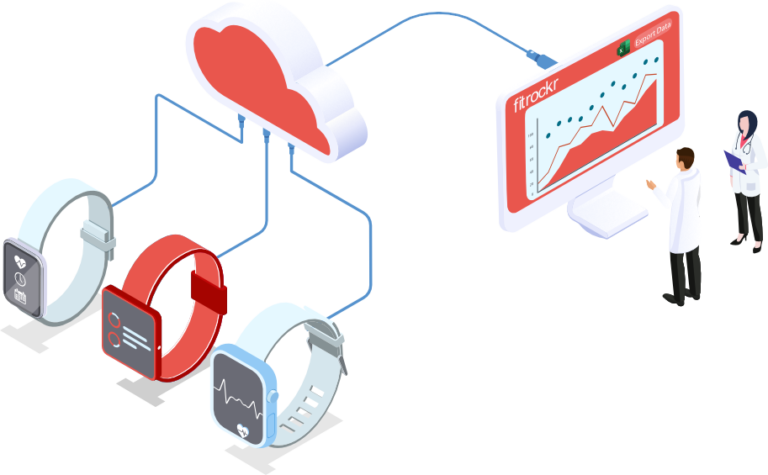
The leading solution for wearable data healthcare & analytics
The world’s most renowned Universities, Hospitals and Medical Institutions run their healthcare projects and research studies on Fitrockr.
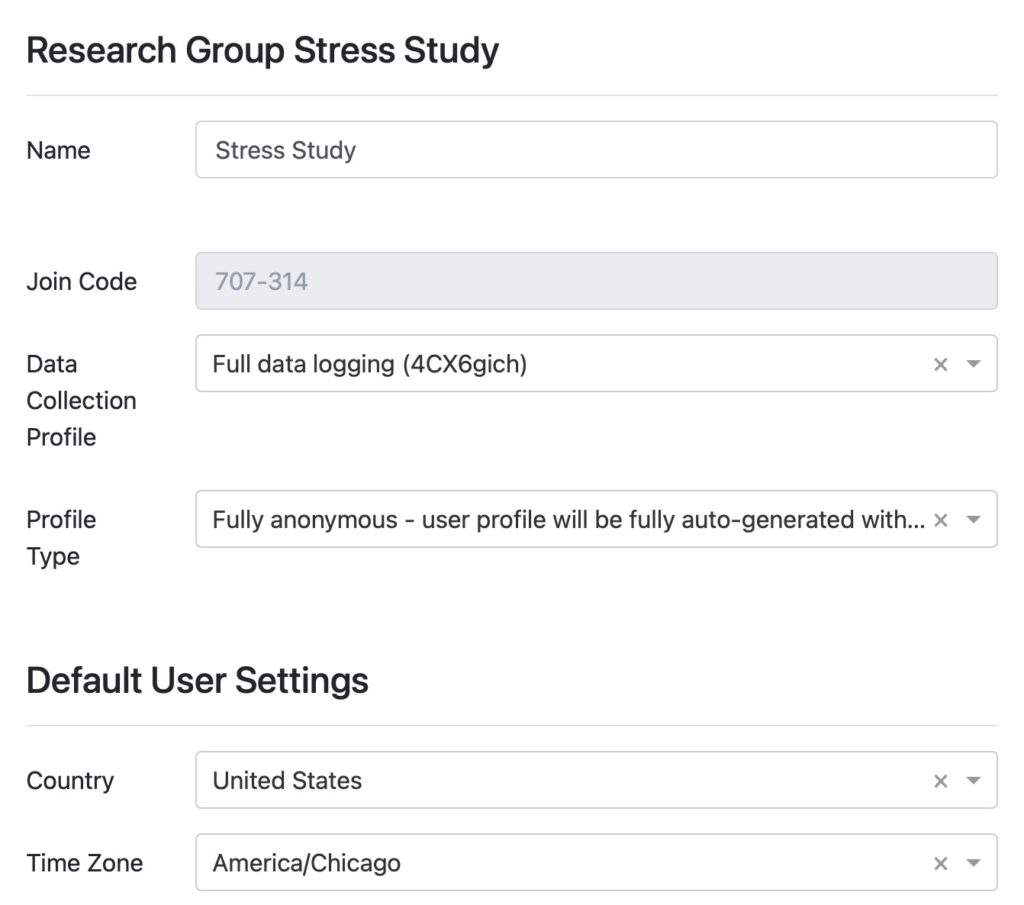
Stress Study
Sleep Study
Epidemiology Study
Activity Study
Validation Study
Clinical Trial
Psychology Study
Mental Health Study
Physiology
Study
use Fitrockr
Conducted
PARTICIPANT MANAGEMENT
Easy participant management no matter how large the population
Fitrockr provides numerous features to efficiently manage your patients.
- Manual set up of participant profiles by administrator.
- Self-service profile set up by patients.
- Excel file batch patient profiles upload.
- Automated patient profiles creation via Rest-API.
- Group patients by projects and research groups.
- User roles with role-specific access rights
Manual setup
Self-service setup
Excel batch upload
Rest-API setup
Flexible grouping
User roles
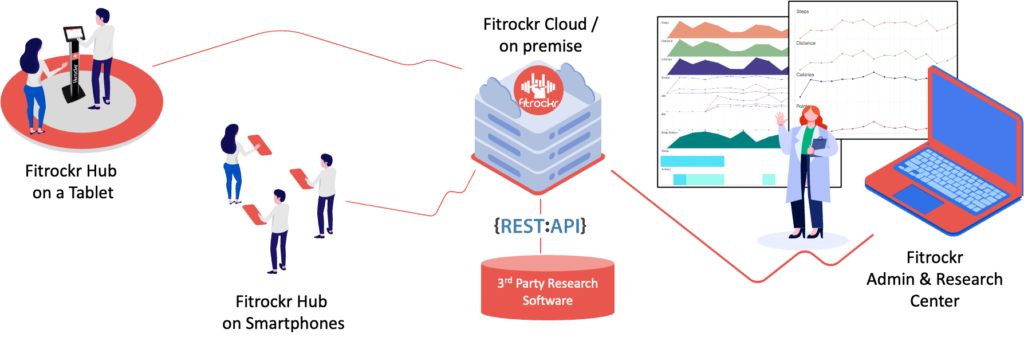
DATA COLLECTION
Plug-and-play solution to collect any data recorded on a Garmin wearable. It cannot be easier.
Fitrockr is a plug-and-play healthcare and research solution to collect any data recorded on a Garmin wearable. Patients simply wear the Garmin device and regularly synchronize it wirelessly through Bluetooth on their smartphone or via a central tablet. Large data amounts can also be uploaded via usb if needed.
Step 1 - Select Garmin device most suitable for your project
Garmin offers a wide range of device models covering all possible research use cases. Decide based on supported data types, storage and battery duration. We help you to find the most suitable device whether you purchase through us or directly at Garmin.

Fitrockr is a corporate solution partner of Garmin since 2018. Fitrockr is one of the Garmin deepest integrated solutions on the market.

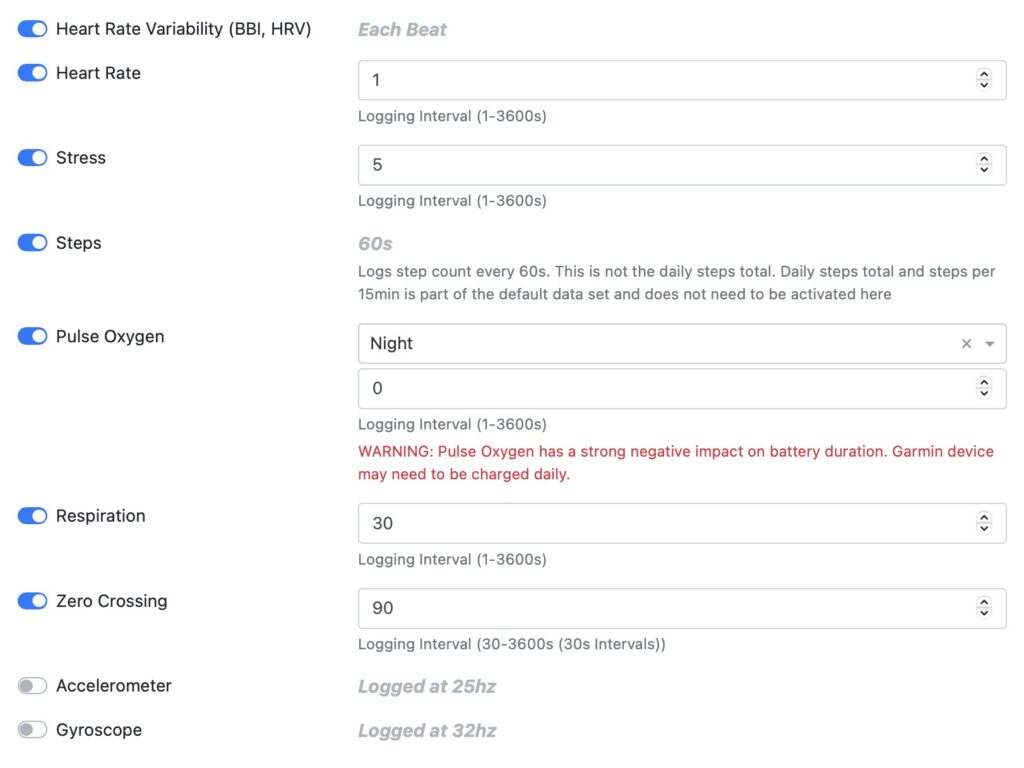
Step 2 - Set data sampling rate
Customize the sampling rate for recorded data flexibly by research group or individual user level.
Totals for each day such as average heart rate, maximum/minimum stress levels, total steps, calories burned, etc.
Sampling rate: Daily
Details for each recorded activity such as GPS information, distance, speed heart rate, temperature, steps per minute, etc.
Sampling rate: 1-5s
Daily totals split into 15 minute epochs with information on steps, distance, met value, stress, heart rate, motion intensity, etc.
Sampling rate: 15 minutes
Break down of sleep duration and sleep phases.
Sampling rate: Seconds
Heart rate variability (HRV/BBI) is the time between each individual heartbeat.
Sampling rate: Millisconds
Heart rate data based on each individual heart beat.
Sampling rate: 1-3600s
Stress level and body battery.
Sampling rate: 1-3600s
Pulse oxygen measured by night or 24/7.
Sampling rate: 1-3600s
Breathing rate.
Sampling rate: 1-3600s
Number of times the signal crosses the zero axis. The acceleration signal measures movement frequency.
Sampling rate: 30-3600s
Accelerometer measures linear acceleration.
Sampling rate: 25hz
Gyroscope helps indicate orientation.
Sampling rate: 32hz
Special set of activity metrics.
Sampling rate: Seconds
Step 3 - Connect and sync Garmin device
Choose from a variety of synchronization use cases, either via the patient’s smartphone or via a central tablet. This covers all current and future research project demands.
The “Visit” Sync Use Case
The “Visit” use case is described by the fact that patients regularly visit one or more central sites to sync their Garmin device.

The “Home” Sync Use Case
The “Home” use case is described by the fact that patients are distributed and sync their Garmin device with their own smartphone at home.
The “Letterbox” Sync Use Case
The “Letterbox” use case is described by the fact that patients are distributed and send their Garmin device to a central site for syncing.
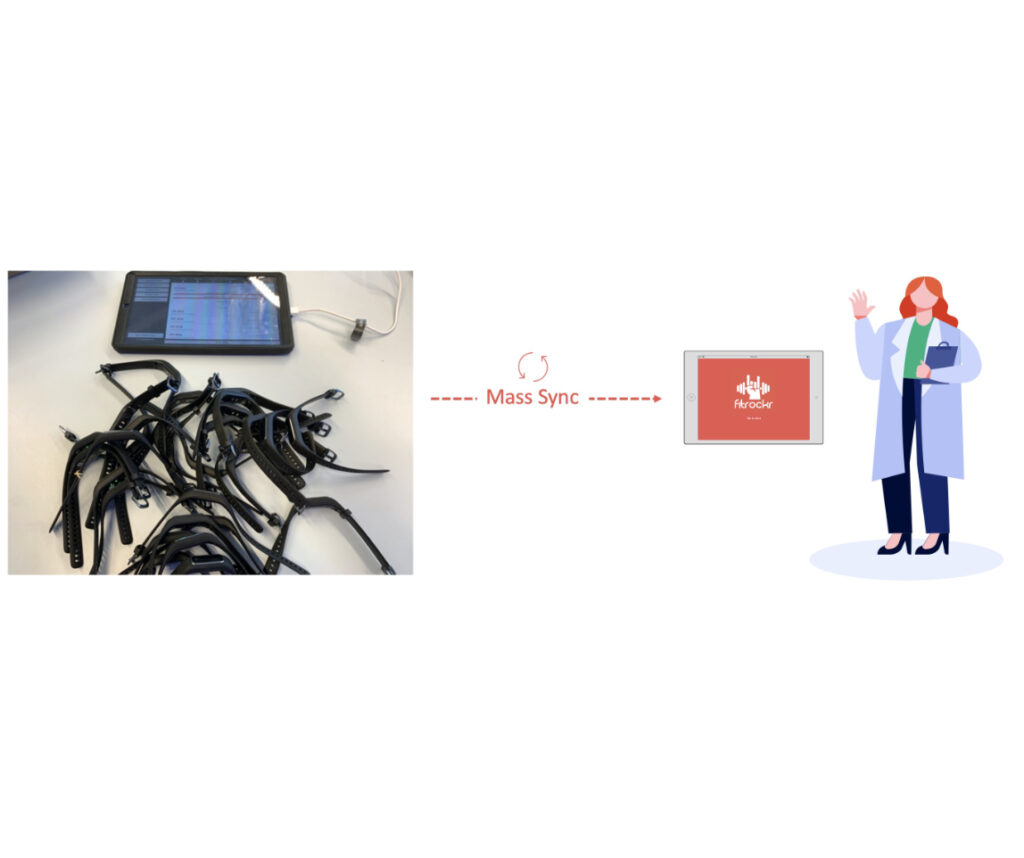
The “Community” Sync Use Case
The “Community” use case is described by the fact that patients are syncing via a local community terminal.
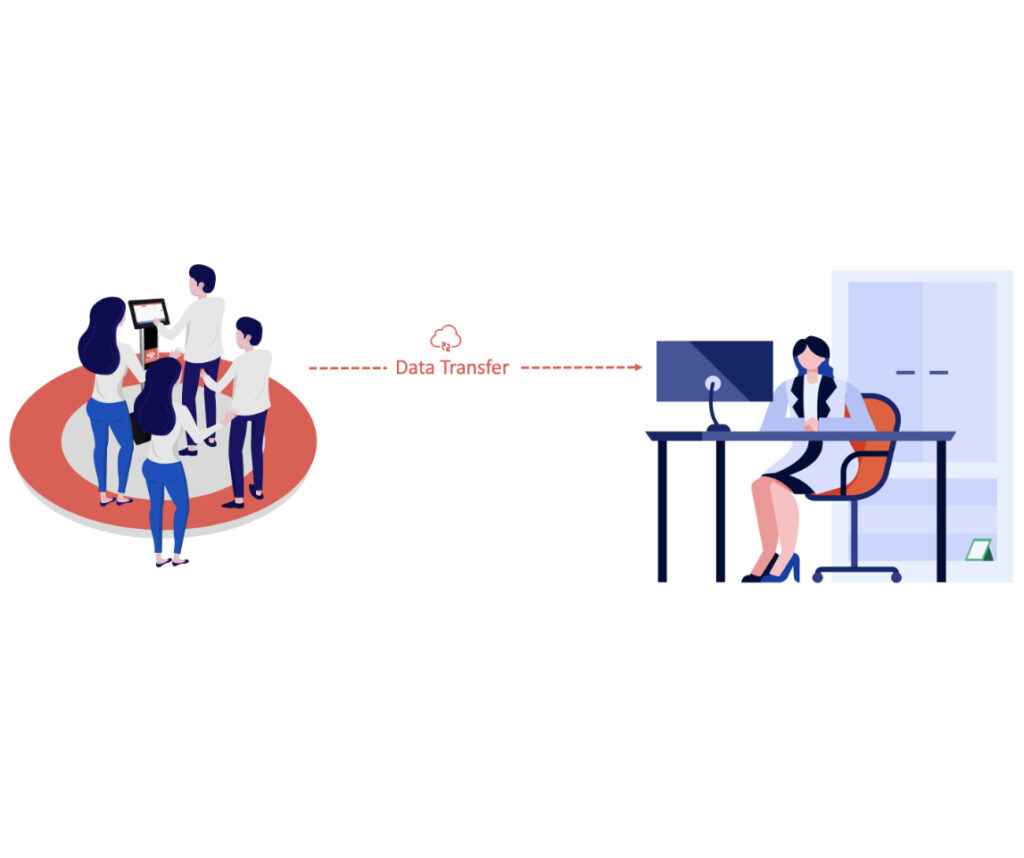
The “Live Streaming” Sync Use Case
The “Live Streaming” use case is described by the fact that study participants are live streaming the data (real-time) from their Garmin device.
MONITORING & ALERTING
Effective monitoring to prevent data gaps during data collection
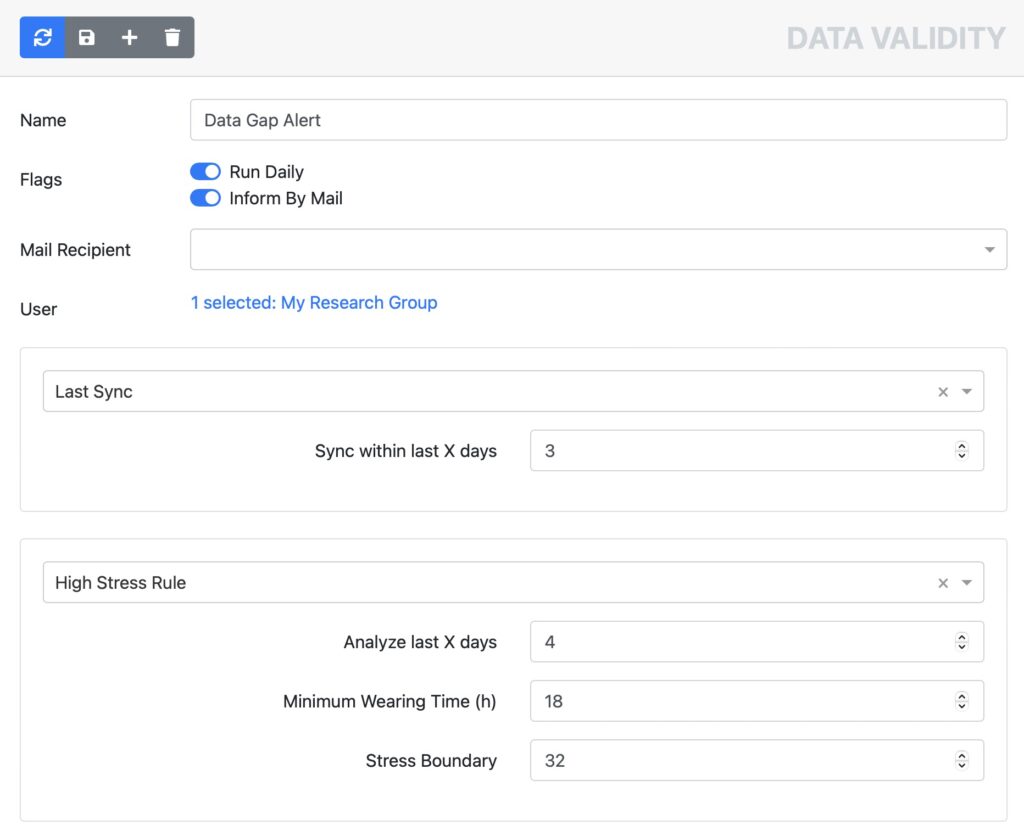
Fitrockr provides effective and automated monitoring tools to ensure that your patients adhere to the data collection process. No more data gaps due to empty batteries or wearables forgotten to wear.
Empty battery alerts
Data gap alerts
Real time data monitor
Data deviation alerts
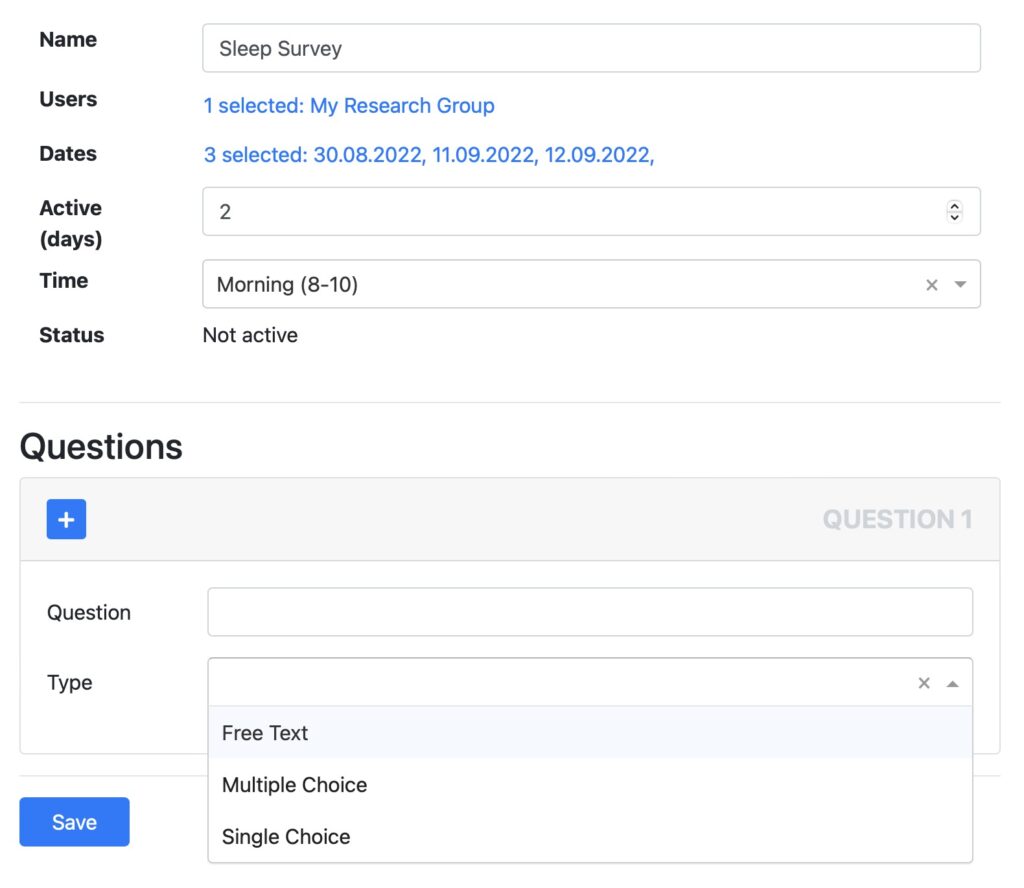
QUESTIONNAIRES & SURVEYS
Collect patient feedback and combine responses with wearable data collected
Setup questionnaires and ask your patients to provide feedback. Questionnaires are automatically displayed in the Fitrockr mobile participant app. Answers can be analyzed and combined with the physiological data collected via the wearables.
- Create free text, multiple choice and single choice questions.
- Set dates when each questionnaire should be answered.
- Schedule notifications to remind participants to provide feedback.
- Analyze questionnaires on screen or download data in Excel.
- Combine feedback with the physiological data collected via the wearables.
Multiple choice
Single choice
Free text
Schedule surveys
Schedule notifications
Export feedback
TASKS & GOALS
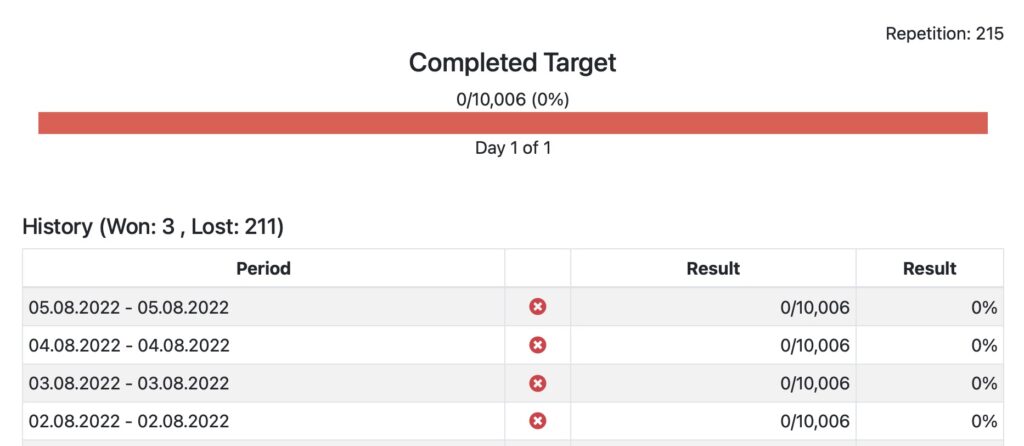
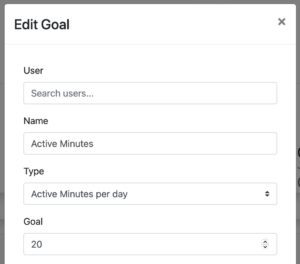
Assign goals to patients and track achievements
Define goals based on steps, distance, calorie burn or activity minutes. Assign goals individually to patients or patient groups and track achievements. Goals are automatically displayed in the Fitrockr mobile patient app.
Step Goal
Distance Goal
Activity Minutes Goal
Calorie Burn Goal
DATA ANALYSIS
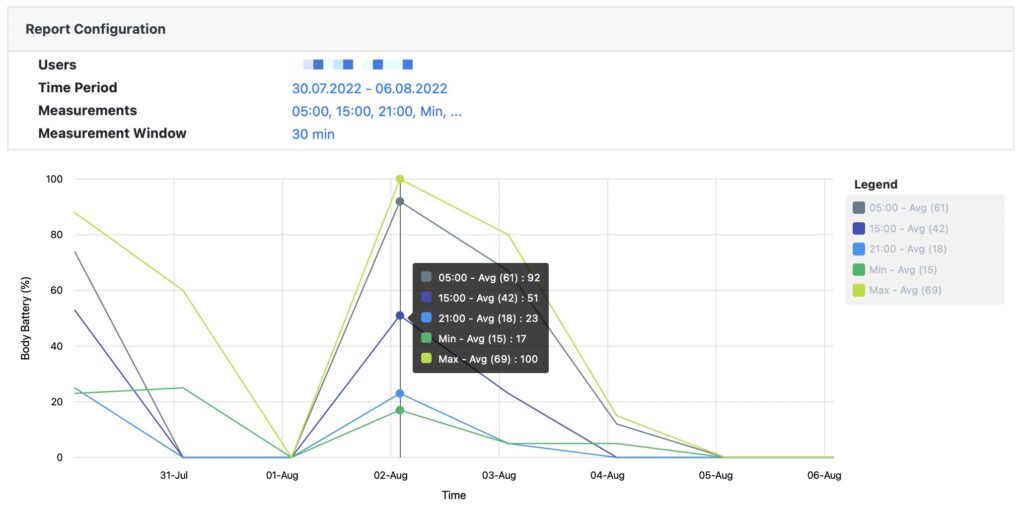
Access, analyze and export high resolution raw data.
All recorded data can be accessed and analyzed via pre-built standard reports or downloaded as Excel or JSON file format.
Pre-built standard reports
Access pre-built standard reports to assess patients’ physiological data. All reports can be viewed on screen or downloaded as Excel file.
If standard reports do not cover your needs, we can build a custom report or just download all raw data and build a report in Excel or via a third party research tool.
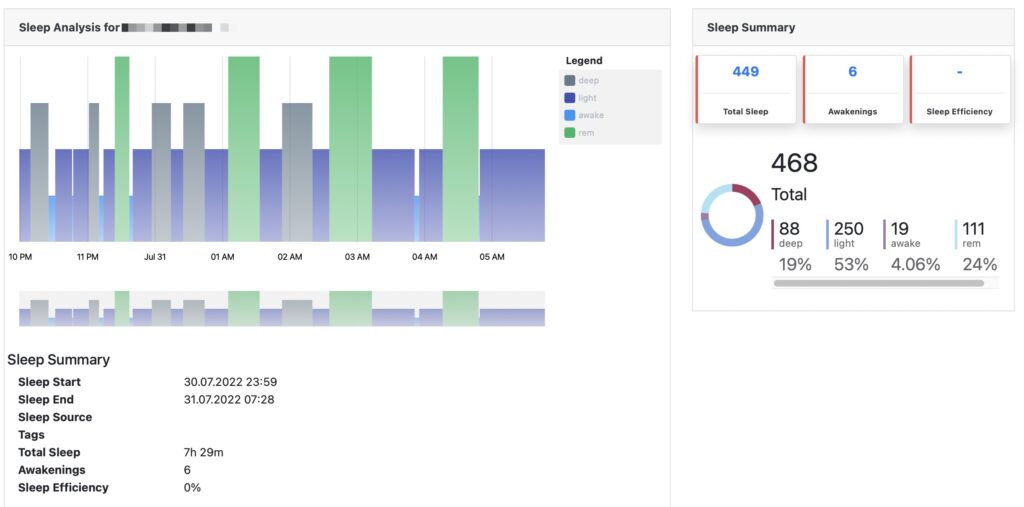
High resolution raw data export
Select timeframe, patients and data types and export any data into Excel or JSON format for further analysis.
Flexible Participant Selection
Flexible Timeframe Selection
Excel Export Format
JSON Export Format
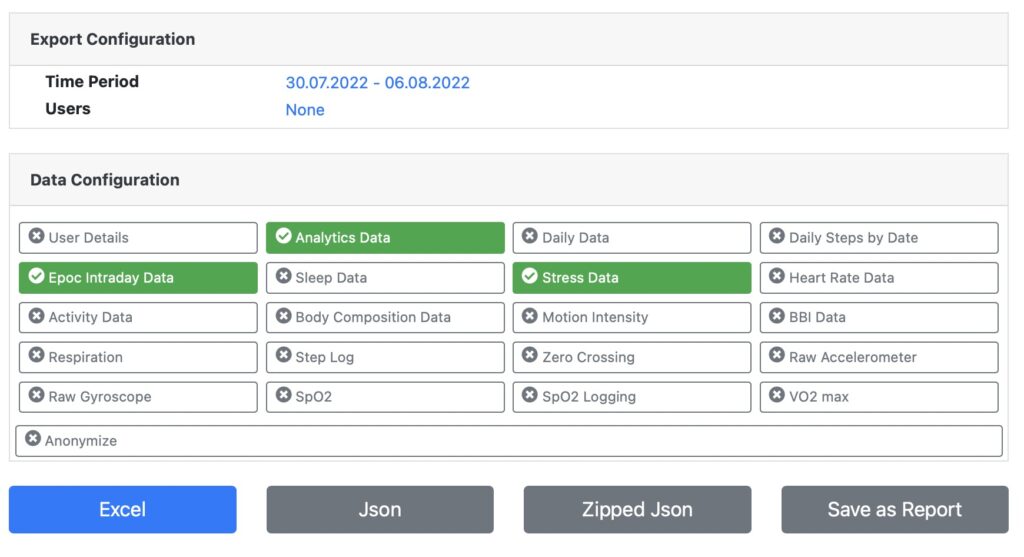
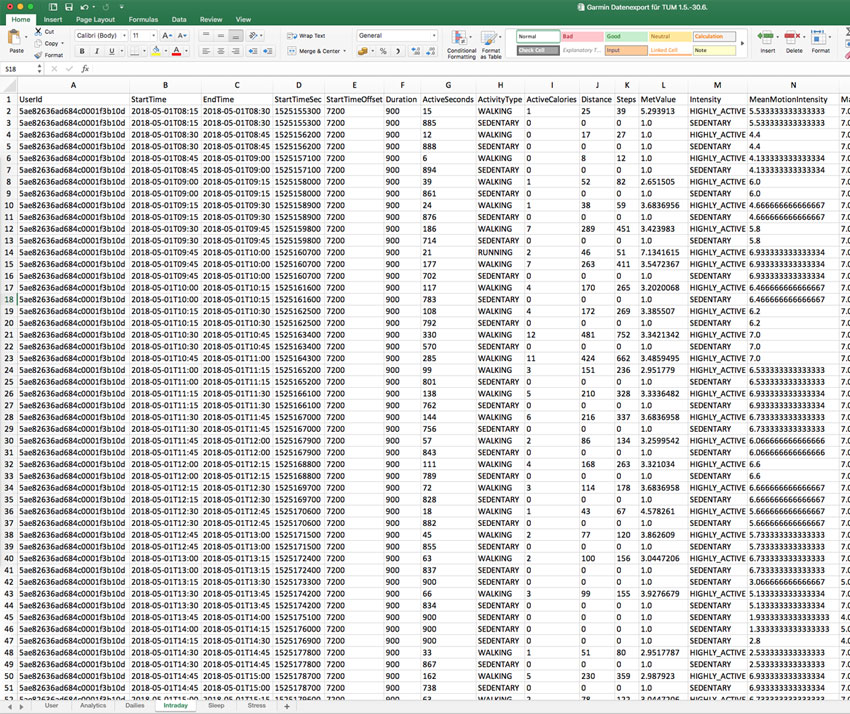
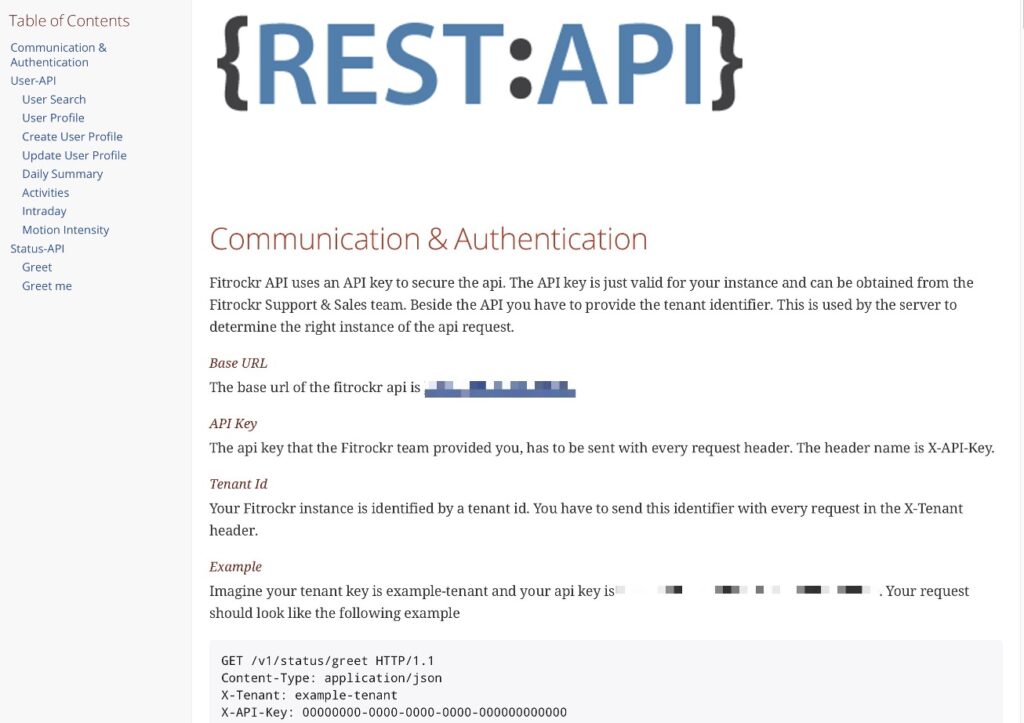
Rest API
Fully automate data synchronization with API Developer Access
Use our Rest-API to automate data exchange. Create new users via Rest API and import any data recorded into your own database.
SECURITY & DATA PROTECTION
Data protection and security tailored to sensitive data research projects
Fitrockr provides secure EU cloud or local on-premise data storage compliant with the latest security and data protection regulations.
- Cloud servers located in Germany under EU data protection law.
- ISO-certified cloud hosting centre.
- Local on-premise installation and hosting available.
- Healthcare analyses can be conducted personalized, pseudonymized or anonymized.
- Each healthcare project receives its own server instance which will be completely deleted including all data at the end of the project.
- Fitrockr is a sole software service provider without any business or intentions in any data. Data is only used for the project purpose.
EU cloud
On-premise hosting
GDPR compliant
ISO certified
GCP compliant
HIPAA certified

ISO
Our Cloud hosting center is ISO/IEC 27001 certified and follows international standards on how to manage information security.

GDPR
We comply to GDPR – the General Data Protection Regulation on data protection and privacy in the European Union and the European Economic Area.

HIPAA
We comply to HIPAA – the Health Insurance Portability and Accountability Act that provides data privacy and security provisions for safeguarding medical information.

GCP
We follow GCP – Good Clinical Practice, an international ethical and scientific quality standard for designing, recording and reporting trials that involve the participation of human subjects.
"
With Fitrockr, we implemented a 360 degree end-to-end healthcare experience at our medical center!
– Medical Institute
"
CONTACT
We serve globally while based in Berlin
Get in touch
Fitrockr (c/o Digital Rebels GmbH)
Friedrichstraße 114 A
10117 Berlin
Germany
© Digital Rebels GmbH. All Rights Reserved.